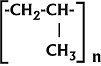
Giulio Natta's original
polypropylene formula - 1954
U l t r a c o n d u c t o r s
- A P r i m e r -
ULTRACONDUCTOR™  n. "Electrical conductors, which have certain properties similar to present-day superconductors.
They are best considered as a novel state of matter."
n. "Electrical conductors, which have certain properties similar to present-day superconductors.
They are best considered as a novel state of matter."
Ultraconductors™ are patented materials being developed for commercial applications. They are made by the sequential processing of amorphous polar dielectric elastomers. They exhibit a set of anomalous magnetic and electric properties, including:
very high electrical conductivity (> 1011 S/cm -1) and
current densities (> 5 x 108 A/cm2) over a wide temperature range (1.8 to 700 K).
Additional properties established by experimental measurements include:
the absence of measurable heat generation under high current;
thermal versus electrical conductivity orders of magnitude in violation of the Wiedemann-Franz law;
a jump-like transition to a resistive state at a critical current;
a nearly zero Seebek coefficient over the temperature range 87 - 233 K;
no measurable resistance when Ultraconductor™ films are placed between superconducting tin electrodes at cryogenic temperatures.
The Ultraconductor™ properties are measured in discrete macromolecular structures which form over time after the processing. In present thin films (1 - 100 micron) these structures, called 'channels', are typically 1 - 2 microns in diameter, 10 - 1000 microns apart, and are strongly anisotropic in the Z axis. RTS, Inc was founded in 1993 to develop the Ultraconductor™ technology, following 12 years of research by a scientific team at the Polymer Institute, Russian Academy of Sciences, led by Dr. Leonid Grigorov, Ph.D., Dc.S. There have been numerous papers in peer-reviewed literature, 4 contracts from the U.S. government and a landmark patent (US patent # 5,777,292). To date 7 chemically distinct polymers have been used to create Ultraconductors™, including olefin, acrylate, urethane and silicone based plastics. The total list of candidate polymers suited to the process is believed to number in the hundreds. In films, these channels can be observed by several methods, including phase contrast optical microscope, Atomic Force Microscope (AFM), magnetic balance, and simple electric contact. The channel structures can be moved and manipulated in the polymer. Ultraconductor™ films may be prepared on metal, glass, or semiconductor substrates. The polymer is initially viscose (during processing). For practical application the channels may be “locked” in the polymer, by crosslinking, or glass transition. The channel's characteristics are not affected by either mode.
A physics model of the conducting structures, which fits well with the experimental measurements, and also a published theory, have been developed. The next step in material development is to increase the percentage or “concentration” of conducting material. This will lead to films with a larger number of conducting points (needed for interposers and other applications) and to wire. Wire is essentially extending a channel to indefinite length, and the technique has been demonstrated in principle. Connecting to these conducting structures is done with a metal electrode, and when two channels are brought together they connect. From an engineering point of view, we expect the polymer to replace copper wire and HTS in many applications. It will be considerably lighter than copper, and have less electric resistance.
 BACK to the "Atypical" page at Superconductors.ORG
BACK to the "Atypical" page at Superconductors.ORG
 BACK to the "Terminology" page at Superconductors.ORG
BACK to the "Terminology" page at Superconductors.ORG