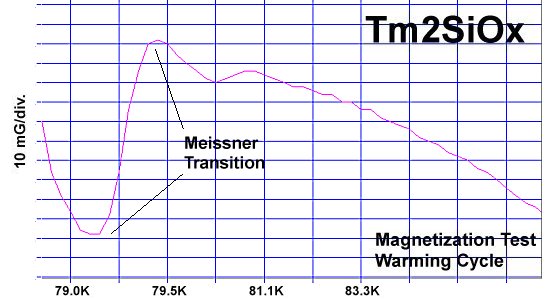
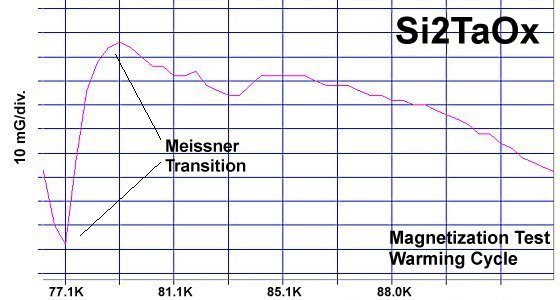


Superconductors.ORG herein reports a "recipe" has been found for making a Meissner transition. It's been discovered that ternary compounds containing two disparate weight metals and oxygen will generate strong diamagnetism at temperatures greater than the boiling point of liquid nitrogen (77K) if the following formula is met:
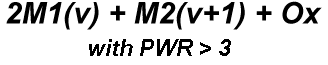
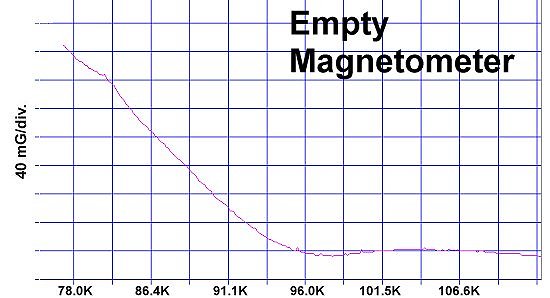
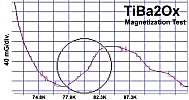
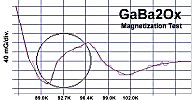
"M1" represents the first metal, of which there are two atoms. "M2" is the second metal, of which there is just one atom. The "v" stands for valency. So, whatever valency M1 has, M2 must be one oxidation state higher. "Ox" is the number of oxygen atoms, which will vary according to the metals' oxidation states and resulting structure that forms. PWR is the planar weight ratio of the heavy-to-light metals' atomic weights (even when the structure is non-planar). Normally this is greater than 3, but can be less if a transition temperature lower than 77K is acceptable. It does not matter whether the heavier metal has the lower or higher oxidation state. This general recipe was initially found to work with divalent/trivalent metals. Now both trivalent/quadrivalent and quadrivalent/pentavalent combinations have also been found to work, so long as their ionic radii are compatible.[1] At page top are magnetization plots of the two latest discoveries. Tm2SiOx, the trivalent/quadrivalent prototype, displays a Tc just above 79 Kelvin. And, above right, Si2TaOx is the quadrivalent/pentavalent prototype, with Tc near 77K. Both materials have Meissner transitions of 80-100 milligauss, suggesting a volume fraction around 40% of the bulk. Below is the plot of the magnetometer when empty. Its trace explains the negative slope on the far left side of both test plots.
Below are plots of the six prototypes that validated divalent/trivalent metals as Meissner transitions as oxides. The original stories that detailed these discoveries are listed on the News page.
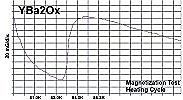
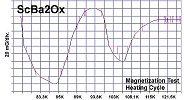
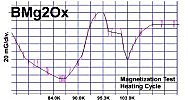
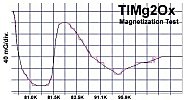
The below stoichiometric amounts of chemicals were used in the synthesis of Tm2SiOx:
Tm2O3 99.99% (Stanford Materials) 14.71 grainsAnd the below amounts of chemicals were used in the synthesis of Si2TaOx:
Ta2O5 99.993% (Alfa Aesar) 11.01 grainsThe chemical precursors were mixed, pelletized at 60,000 PSI and sintered at 880C for 11+ hours. After sintering the pellet was annealed for 10+ hours at 500C in flowing O2. Testing temperatures were determined using an Omega type "T" thermocouple and precision OP77 DC amplifier. The magnetometer employed twin Honeywell SS94A1F Hall-effect sensors with a tandem sensitivity of 50 mv/Gauss.
RESEARCH NOTE: These materials can be strongly hygroscopic. All tests should be performed immediately after annealing.
RE-PUBLICATION NOTICE: Elsevier Publishing, dba Elsevier Science, as well as Morris Communications, both print and broadcast divisions, are specifically prohibited from re-publishing any part of this news story.
E. Joe Eck
© 2016 Superconductors.ORG
All rights reserved.
 BACK to "News" page at Superconductors.ORG
BACK to "News" page at Superconductors.ORG